
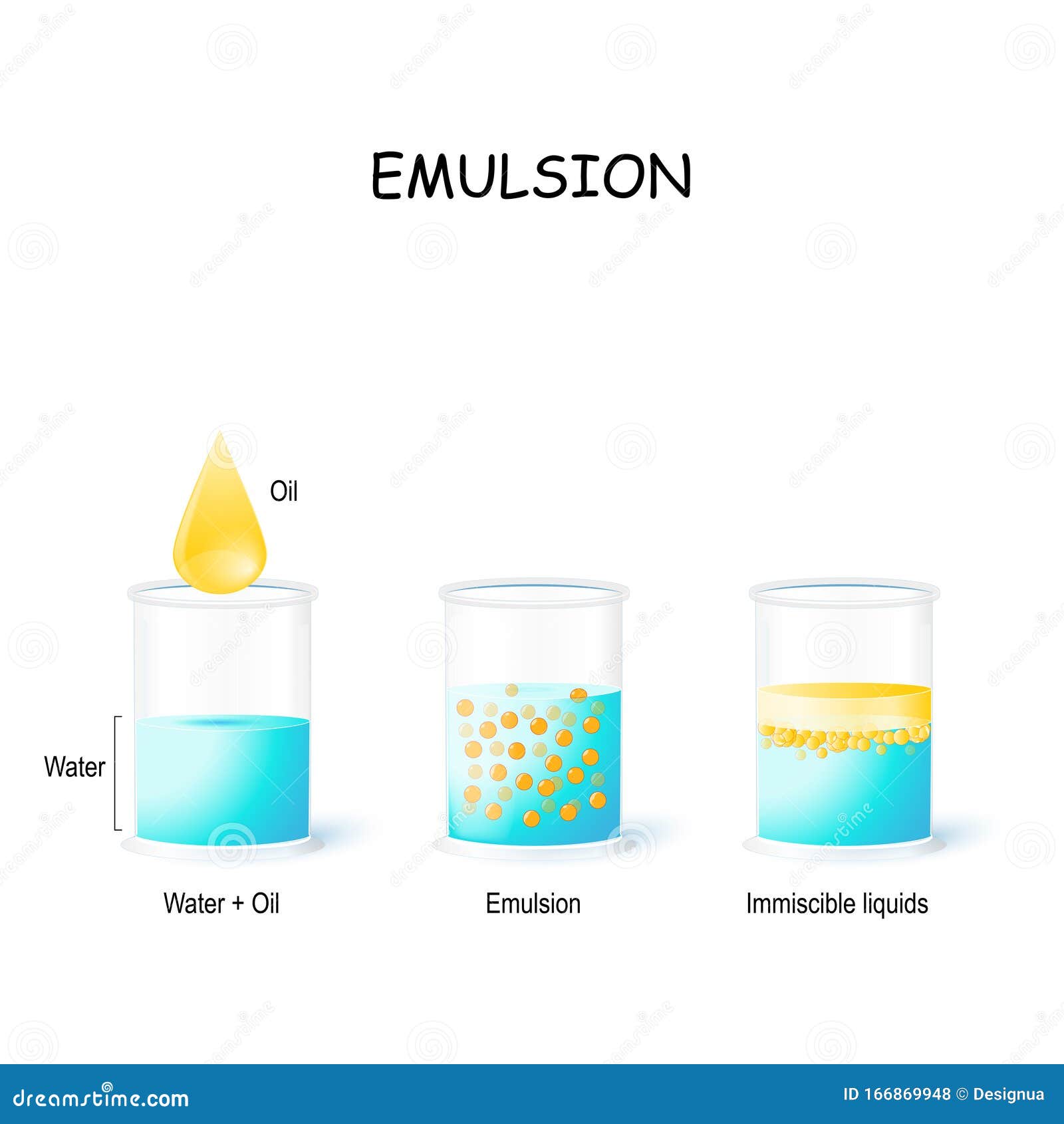
Emulsions are thicker than either the water or of fat/oil they contain, which is a useful property for some foods. By vigorously mixing the emulsifier with the water and fat/oil, a stable emulsion can be made.Ĭommonly used emulsifiers include egg yolk, or mustard. The hydrophilic end of the emulsifier molecule is attracted to the water and the hydrophobic end is attracted to the fat/oil. These help to form and stabilise the emulsions, preventing or slowing the water and fat/oil from separating.Įmulsifier molecules work by having a hydrophilic end (water-loving) and hydrophobic end (water-hating). To prevent the mixture from separating substances called emulsifiers can be added. Other articles where oil-in-water emulsion is discussed: pharmaceutical industry: Liquid dosage forms: Oil-in-water emulsions will mix readily with. The difference between water in oil and oil in water emulsions is water droplets suspended in oil, while oil droplets suspended in water. Sometimes you want to make a thick, rich. Oil and water are normally immiscible, but with proper mixing and stability agents, a permanent mixture, or emulsion, can be achieved. If a certain emulsifier works in your emulsion with 5 oil, it will very probably not be the best choice for another emulsion with 40 oil phase. continuous phase The biggest difference between o/w and w/o emulsions is which phase is suspended and which is continuous. However the mixture is unstable and if you left it for a while it would soon separate out into water and oil layers again. A colloidal system in which one liquid is dispersed in another liquid immiscible with it is called an emulsion. Depending on the concentration of the oil phase (or water phase), you should try to find the most suitable emulsifier for that system. If you shake the oil and water together then the oil breaks up into tiny droplets and becomes distributed in the water forming a mixture.

If you add a drop or two of oil to water you can see that it does not dissolve or combine with the water: the oil floats on the water. These emulsions disperse oil droplets as globules throughout a water-based (aqueous) continuous phase.


 0 kommentar(er)
0 kommentar(er)
